| Abstract: Students learn about the atomic structure and the subatomic particles through dancing. Learning objectives: The subatomic particles: protons, electrons, and neutrons and their structure. Atomic number (Z) and mass number (A). Previous knowledge: Atomic theory, Chemical elements, Chemical compounds Total duration: 45 min |
Materials: pc, projector, coloured papers
PHASE 1 Visualisation
Duration:12 min
Firstly students watch a video named “The Waltz of the Snowflakes” from the famous play “The Nutcracker” performed by The Royal Ballet of London.
Afterwards, students describe what they have seen and they answer to the question: “What is needed to create a choreography?”
Phase 2 Artistic action and experimentation
Duration:10 min
Development:
Students are encouraged to dance in a certain choreography representing the atom of a Lithium isotope while listening to a piece of classical music composed and played by a Greek composer Stefanos Korkolis on the piano.
6 students are in the centre forming a cycle and moving slowly and steadily and 3 other students are dancing around them and around themselves. All of them holding sings of the subatomic particles (protons, neutrons, electrons) and their charge.
Phase 3: Reflexion and debate
Duration: 23 min
Development:
Focus on the lesson which concerns the “Structure of the Atom”.
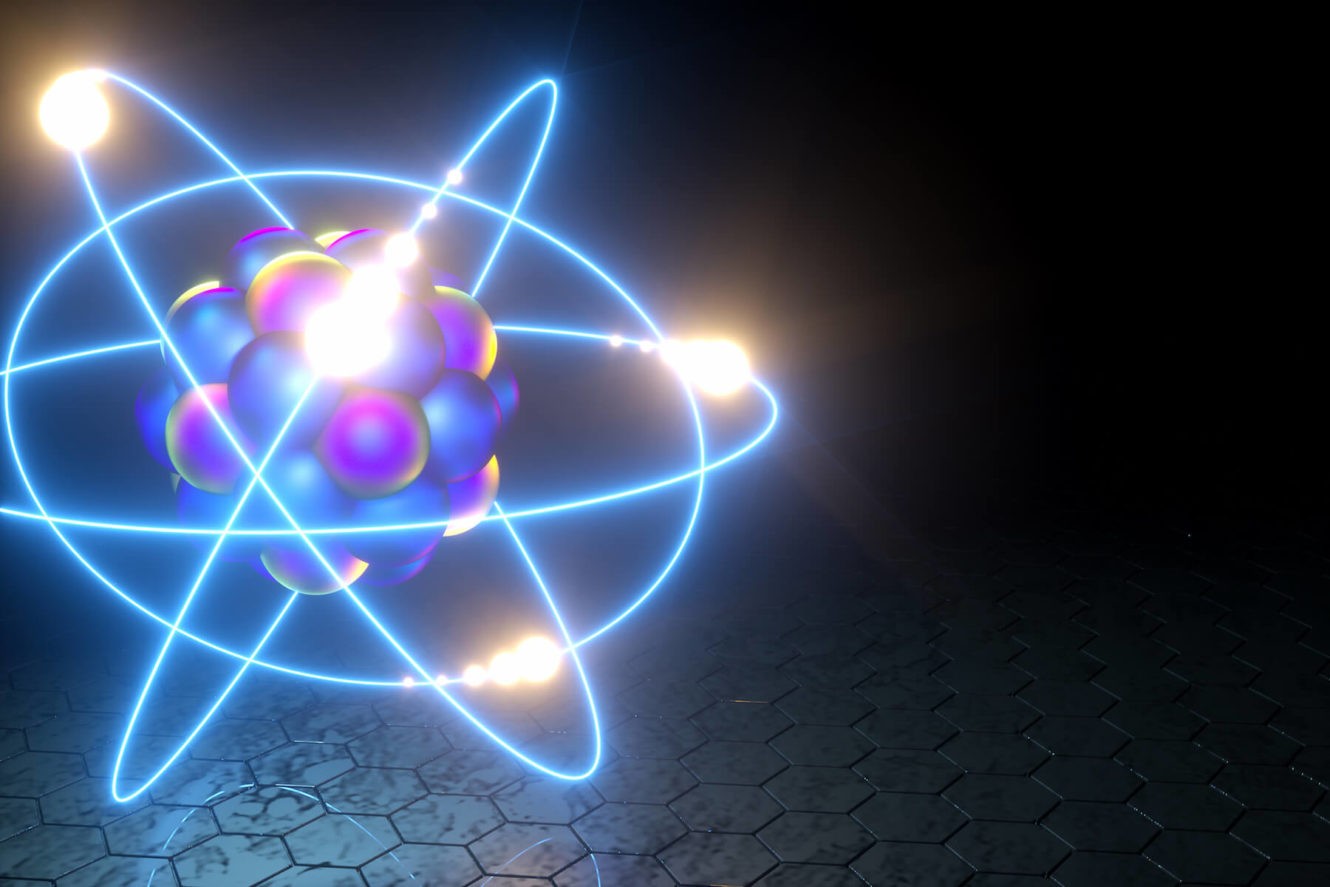
Atomsare the smallest particle of an element that can exist alone.
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged). Atoms have different properties based on the arrangement and number of their basic particles.
References and links: